37 2.36 Phospholipids
Phospholipids are similar in structure to triglycerides, with the only difference being a phosphate group and nitrogen-containing compound in the place of a fatty acid.

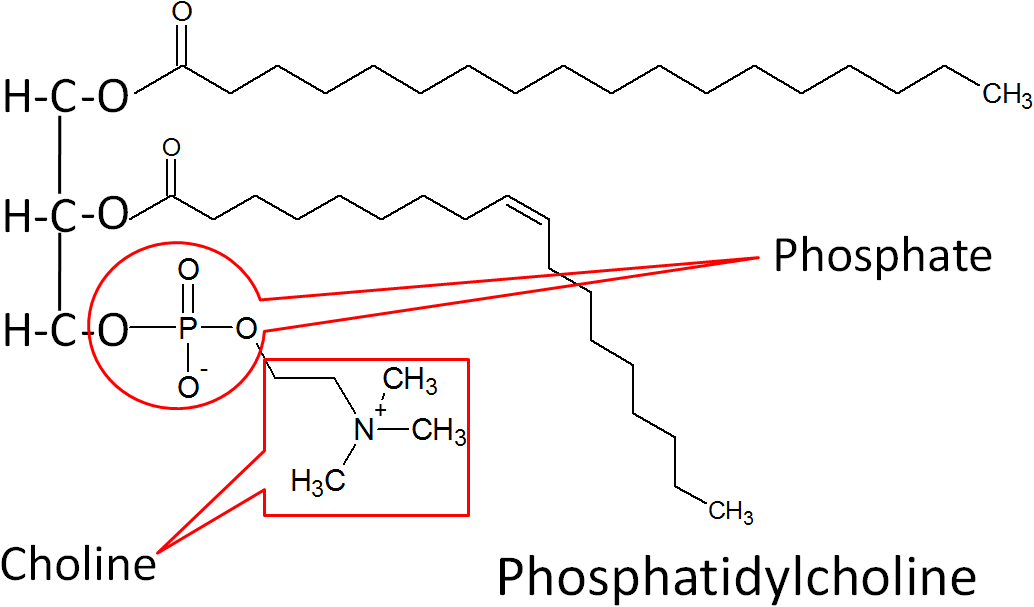
The best known phospholipid is phosphatidylcholine (aka lecithin). As you can see in the structure below, it contains a choline off of the phosphate group.
However, you will not normally find phospholipids arranged like a triglyceride, with the 3 tails opposite of the glycerol head. This is because the phosphate/nitrogen tail of the phospholipid is polar. Thus, the structure will look like the 2 figures below.


Similar to triglycerides, phospholipids are also represented as a hydrophilic head with two hydrophobic tails as shown below.

Phospholipid Functions
Because its structure allows it to be at the interface of water-lipid environments, there are two main functions of phospholipids:
1. Key Component of the Cell’s Lipid Bilayer
2. Emulsification
Number 1 in the figure below is a cell’s lipid bilayer, while 2 is a micelle that is formed by phospholipids to assist in emulsification.

1. Key Component of Cells’ Lipid Bilayers
Phospholipids are an important component of the lipid bilayers of cells. A cross section of a lipid bilayer is shown below. The hydrophilic heads are on the outside and inside of the cell; the hydrophobic tails are in the interior of the cell membrane.

2. Emulsification
As emulsifiers, phospholipids help hydrophobic substances mix in a watery environment. It does this by forming a micelle as shown below. The hydrophobic substance is trapped on the interior of the micelle away from the aqueous environment.

As a result, it can take a hydrophobic liquid (oil) and allow it to mix with hydrophilic liquid (water).

Foods rich in phosphatidylcholine include: egg yolks, liver, soybeans, wheat germ, and peanuts8. Egg yolks serve as an emulsifier in a variety of recipes. Your body makes all the phospholipids that it needs, so they do not need to be consumed (not essential).
References & Links
1. http://en.wikipedia.org/wiki/File:Phospholipid.svg
2. http://commons.wikimedia.org/wiki/File:Popc_details.svg
3. http://en.wikipedia.org/wiki/File:Phosphatidylcholine.png
4. http://en.wikipedia.org/wiki/File:Lipid_bilayer_and_micelle.png
5. http://en.wikipedia.org/wiki/File:Bilayer_hydration_profile.svg
6. https://en.wikipedia.org/wiki/Micelle#/media/File:Micelle_scheme-en.svg
7. http://en.wikipedia.org/wiki/File:Emulsions.svg
8. Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw’s perspectives in nutrition. New York, NY: McGraw-Hill.

